Computational Materials Science Center
College of Science
George Mason University
SAMP: Structure-Adaptive Materials Prediction
Overview | Highlight | Cloud Computing | People |
Zeolites are very interesting crystalline compounds that in some cases occur naturally - but also have been synthesized - and contain voids in the crystal structure that allow facile movement of water and ions throughout the crystal structure. The sustained interest in zeolites from the materials science and chemistry communities is based on their unique ion-exchange ability as well as their abilities to allow substances of certain sizes into their voids. Zeolites are widely used for water softening, separation and removal of gases and solvents, fuel refining and petrochemical cracking. They can actually screen molecules of different size since the voids are of such a size as to allow certain molecules to freely travel through the matrix while larger molecules move through the crystal with much more difficult. This process of selectively allowing molecules of varying size is called "molecular sieving" and is relatively unique to this class of molecules and is one of the properties that make them so valuable in the chemical sciences.
Researchers are looking for cost-effective solutions to produce targeted zeolites, which could replace energy-inefficient separations (such as in fuel refining) with membrane-sieves separations. For example, new zeolite membranes could adsorb undesired chemicals dissolved in a fluid simply by passing it through the sieve instead of heating the feedstock liquid and distilling the desired chemicals.
The properties of zeolites were learned over a fairly long period of time. The first zeolite was discovered in 1756 by Alex Cronstedt, when he discovered them in small cavities of volcanically derived rock. He discovered that this mineral had the capacity to store water, which could be removed by mild heating. Since then zeolites have been discovered in saline alkaline lakes, soils and land surfaces, in marine deposits, flowing systems and in other areas around the world . (Dyer, A. 'An Introduction to Zeolite Molecular Sieves', John Wiley, New York, 1988, pgs. 2-9)

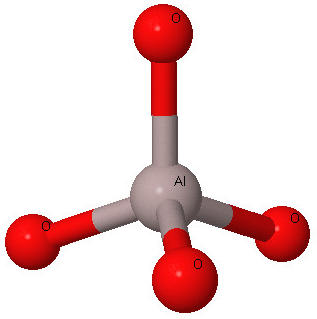
Zeolites are aluminosilicate minerals that have a ratio of oxygen to aluminum and silicon of 2 to 1. Within a zeolite crystal silicon (or aluminum) and oxygen atoms are arranged in tetrahedral [SiO4]4- and [AlO4]5-groups. Repeated three dimensional arrangements of these polyhedra connected together by sharing their oxygen with other polyhedra gives rise to the zeolite crystal structures. Over different 130 framework arrangements of the tetrahedral groups are known. In addition to having silicon or aluminum as the tetrahedral atom, other chemistries have been synthesized, such as the microporous aluminophosphates, known as ALPOs. (Further details click here).
The compounds listed on left handside of this page are Zeolite-L structures. The link for each compound provides a jmol visualisation of the unit cell and a supercell for each compound as generated by the tools set under development as part of this project. The raw data for these compounds was collected from the American Mineralogist Crystal Structure Database.
Inherent in the very regular arrays of polyhedral super-lattice frameworks are large vacancies, pores. It is the arrangement of these elements that favors a network of channels and pores that looks like a honeycomb structure. The pore sizes are roughly 3 to 10 Å in diameter. The negatively charged [SiO4]4- and [AlO4]5-- groups that that form the channels and pores within a zeolite structure attract positively charged ions. The major ion exchangers of zeolites are sodium, calcium, barium, and potassium, although depending on the given lattice structure water, ammomium and other charged molecular entities are found absorbed within zeolite channels and pores The porous walls in zeolites are ideal surfaces for chemical reactions. In fact, one gram of zeolite gives several thousand square feet of surface area! Because of this property, zeolites have a great absorbing power.
An additional ability of zeolites is to exchange cations. This ionic substitution capability allows for selective adsorption of harmful elements from water, air, soil, or other liquids. For example zeolites are used for the removal of calcium from hard water because they exchange sodium ions for calcium ions and the result is soft water. Zeolites have strong affinity for certain harmful heavy metals such as lead and chromium. One example of this ability is their use to clean landfills and to clean industrial sites because they prevent the release of unwanted elements into the environment. Zeolites can absorb acids, ammonia, bleach, blood, diesel, gasoline, insect killers, mold, mildew, oils, paints, turpentine, and urine, among others.
Zeolites commercial, medical, and environmental applications are very vast and very inexpensive. They are used in paper products, concrete products, talc products, pollution, hazardous waste clean-up, acid rain, lead contamination, oxygen purification in cars, run off, overflowing landfills, and waste treatment.
The synthetic chemist would benefit from having predictions on how to modify these naturally occurring zeolites and tailor the production of new ones that are functionally viable based on the energetic and structural properties.